
Powerful free webinars from PCQACL’s POWER series
18 July 2022Last year, one of the successful projects of PCQACL was the launch of PCQACL Academy Online Webinar Series or P.O.W.E.R. which aimed to provide free continuing medical education in the field of laboratory medicine. This year, POWER continued to do this through the help of industry partners. Here’s a recap of the 2022 series:

POWER 5: Philippines’ Cell Morphology Day (in partnership with Sysmex)
By Michael Vincent Soon, MD, MBA
Last April 20, 2022, the PCQACL online webinar series presented a lecture on Hematologic Malignancies by Dr. Francisco Tria IV and a lecture on Red Cell Morphology Anomalies cases by Dr. Alejandro Arevalo.
Prior to the actual lectures, registered participants could participate in a prelecture activity by which was made using technology by Sysmex and Cellavixion. For the activity, digital blood smears were provided to participants where they would sort different cells by morphology and identify and grade red blood cells with abnormal morphology. Participants were asked to submit their answers which were then discussed at the start of Dr. Tria’s lecture.
Dr. Tria’s lecture began with a short discussion on the prelecture activity, where he discussed common mistakes made by participants and how to properly evaluate blood smears. He then proceeded to discuss five different cases of patients with hematologic malignancies, together with their blood smears. As each case was presented, Dr. Tria discussed the features you would commonly see in blood smears in each of the five conditions, and how to differentiate them from common mimics. After the features have been identified, Dr. Tria then discussed the next steps to take and how to correlate them with the patient’s clinical presentation, CBC, and other laboratory tests. At the end of the lecture, there was a short question and answer session.
Dr. Arevalo’s lecture similarly began with a short discussion on the prelecture activity, followed by a review on normal red blood cell morphology, which included a review on red blood indices, what they measure, the methods through which they are measured, and their correlations to common red blood cell conditions, such as iron deficiency anemia and thalassemia. Like the first lecture, Dr. Arevalo’s lecture also revolved around five cases of patients with different conditions with altered red blood cell morphology. This included in this discussion were how various abnormal red blood cells arise, how to distinguish them from common mimics, as well as the differences in their pathophysiology. An example of this was how to differentiate bite cells from blister cells. Also discussed were how to correlate these findings with the patient’s clinical presentation, and other available laboratory data.

Dr. Edna Mae Lasap-Go, PCQACL Board of Trustee, moderator of the webinar together with the first speaker Dr. Francis Tria IV during the Q and A portion

Dr. Edna Mae Lasap-Go, PCQACL Board of Trustee, moderator of the webinar together with the first speaker Dr. Alejandro E. Arevalo during the Q and A portion
Ms. Josephina Soriano, RMT, PCQACL Board of Trustee (left) was the master of ceremonies while Dr. Sarah Jane L. Datay-Lim, PCQACL President (right) gave the opening remarks
POWER 6: Transitioning into the New Normal (in partnership with JARC Group)
By Louise De Guzman, MD
The Philippine Council for Quality Assurance in Clinical Laboratories (PCQACL) in partnership with the JARC Group of Companies Affiliates MEDTEK and MAN PHARMA held its online webinar educational series entitled Transitioning into the New Normal last May 31, 2022. Moderated by Dr. James Peter Aznar and Mr. Benzon Pisico of the PCQACL Board of Trustees, and led by Ms. Debbie Sardinia, RMT, Chief Medical Technologist of The Medical City Clark, the program had three illustrious speakers, each tackling different aspects of assays that can be used in our New Normal. Dr. Sarah Jane L. Datay-Lim, FPSP, PCQACL's current President, started the event with a heartfelt welcome to the attendees, followed by Dr. Tze Wei Poh and her presentation on "Utilizing COVID panel testing as a new algorithm and beyond."
She introduced her topic with a brief discussion on the mechanism of SARS-CoV-2 entry within human cells, the immune response to the virus, and how the detection of neutralizing antibodies can benefit clinical practice and public health. Public health-wise, a significant rise in antibodies from baseline to convalescent phase allows for retrospective case confirmation, while sero-surveillance studies can be used to support the investigation of an ongoing outbreak.
In this regard, several assays have been made to detect levels of circulating neutralizing antibodies. One such qualitative assay is the Access SARS-CoV-2 IgG which detects antibodies to the viral Spike proteins. It is a two-step assay which can give results in 25 minutes. A quantitative version is also available, called the Access SARS-CoV-2 IgG (1st IS) Assay, which is a fully quantitative assay traceable to the first WHO International Standard Anti-SARS-CoV-2 Immunoglobulin, thereby allowing for the accurate calibration of assays and reduction of interlaboratory variations.
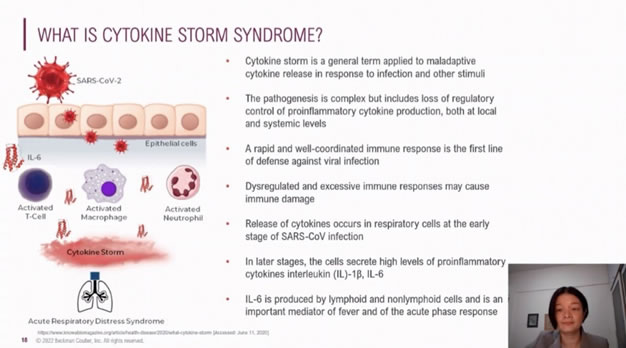
Dr. Tze Wei Poh also discussed other biomarkers which have been identified as important for the management of patients with COVID19. A subgroup of patients with severe symptomatology have Cytokine Storm Syndrome (CSS), which is caused by a maladaptive cytokine release in response of infection due to the loss of regulatory control of proinflammatory cytokine production, leading to a dysregulated immune response. One such biomarker is IL-6, and the immediate initial evaluation of IL-6 levels can identify patients who are at risk for severe respiratory distress. IL-6 can be used as an early indicator of CSS, and a statistically significant predictor of clinical deterioration.
Aside from IL-6, other useful biomarkers include Procalcitonin and Troponin, which also directly correlate with patients who are at higher risk for severe infection.
The next speakers were Dr. Eric Morreale and Mr. Christopher Cotreau on "Elevating quality: Assay Verification, Calibration, and Linearity for SARS-CoV-2 Testing," which tackles the importance of assay verification and calibration when an approved test method is introduced to the laboratory.
Verification involves the confirmation that the laboratory can replicate the manufacturer’s performance claims following the package insert. Performance specifications involve accuracy (test results falling within the manufacturer’s stated acceptable limits) and precision (confirmation that the laboratory can produce comparable results regardless of various conditions).
He discussed several different types of prepackaged panels with diverse demographic information and highly characterized data as well as comprehensive data sheets. For diagnostic manufacturers, the AccuSeries Panels can expedite assay development and regulatory submission. For clinical laboratories, these panels can determine the assay sensitivity, specificity, and reproducibility as well as aid in operator training and competency.
Meanwhile, calibration verification is a regulatory requirement for laboratories as government by the Clinical Laboratory Improvement Amendments (CLIA) regulations or the ISO 15189 requirements for quality and competence. It is performed by establishing acceptance limits; choosing materials for the experiment; running samples in duplicate or triplicate; performing data reduction and statistical analysis; and reviewing and approving experimental results. One such VALIDATE product is the MSDEx (R), a data reduction software program used to analyze linearity and calibration results. It is multi-use and has an open-vial stability, and can analyze at least 165 analytes of clinical interest, including but not limited to general chemistry, lipoproteins, and glycosylated hemoglobin.
Dr. Tze Wei Poh and her presentation on "Utilizing COVID panel testing as a new algorithm and beyond.

Mr. Benzon Pisico, PCQACL Board of Trustee was the moderator for the first lecture.

Snippet from Dr. Eric Morreale and Mr. Christopher Cotreau’s lecture on "Elevating quality: Assay Verification, Calibration, and Linearity for SARS-CoV-2 Testing"

Dr. Peter Aznar, PCQACL Board of Trustee was the moderator for the second part of the webinar and facilitated the live Question and Answer portion.

Ms. Debbie Ann Sardinia, RMT (left), Chief Medical Technologist of The Medical City Clark was the master of ceremonies; Dr. Sarah Jane L. Datay-Lim (right), PCQACL President gave the opening remarks.
POWER 7: Taking a leap towards urinalysis standardization and quality improvement (in partnership with Mindray)
By Sarah Jane L. Datay-Lim, MD, FPSP
Urinalysis was the highlight for POWER 7 entitled “Taking a leap towards urinalysis standardization and quality improvement” last June 30, 2022, in partnership with Mindray. The invited speaker for the webinar was Dr. Niu Wenyan who specializes in Immunology and Molecular Biology, the laboratory director at the Hospital of Chengdu University of Traditional Chinese Medicine. The webinar was moderated by PCQACL board of trustee, Dr. Bernadette R. Espiritu and master of ceremonies was Mr. Boylyn Cezar, RMT, chief medical technologist from Manila Med.
The webinar kicked off with the presentation from the sponsor, Mindray, followed by welcome remarks by PCQACL president Dr. Sarah Jane L. Datay-Lim. This particular webinar theme was aligned with the upcoming project of PCQACL Committee on Education Training and Research (CETR) to standardize urinalysis through publication of a monograph similar to the previously published monograph on CBC standardization in 2016. It aimed to increase awareness about urinalysis since almost all laboratories perform this test and there is a need to improve the quality and standards in reporting.
Dr. Wenyan covered the basics of urinalysis which included the procedures and important aspects of the test. The physiology behind the formation of urine as well as the important findings were highlighted for correlation. This discussion formed the foundation for the of urinalysis that helped the participants understand better the urinalysis findings during the rest of the webinar. The physical and chemical evaluation of urine was reviewed, together with the principles of testing. The different crystals and sediments were extensively discussed together with some photographs for better visualization and understanding. The different strategies for quality improvement were also mentioned by the speaker which gave the participants vital information on how they can improve the performance of the test in their practice. The last part of the lecture featured the different types of automation that is currently available and its role in improving the quality of urinalysis.
During the Question and Answer portion, Dr. Espiritu asked about the External proficiency program because it was discussed during the lecture. It was highlighted that in Dr. Wenyan’s laboratory, they participate in this type of program for quality improvement, particularly in their chemical and microscopic examination of sediments. On a last note, automation can significantly improve quality of urinalysis but without this technology, steps can still be made to ensure high quality and standards are met through continuous improvement of technique, policies for review and recheck of abnormal results. Mr. Cezar closed the webinar after a short presentation from Mindray.

Dr. Niu Wenyan, speaker for POWER 7 discussed “Taking a leap towards urinalysis standardization and quality improvement
Dr. Bernadette Espiritu, PCQACL Board of Trustee was the moderator for the webinar

Dr. Sarah Jane L. Datay-Lim, PCQACL president gave the opening remarks at the start of the webinar

Mr. Boylyn Cezar, RMT, chief medical technologist from Manila Med was the master of ceremonies
